Difference Between Form FDA 483, Warning Letters and EIR

This article describes about difference between Form FDA 483, Warning Letters and EIR and its systemic schematic flow chart for better understanding.
What is mean by Form FDA 483 ?
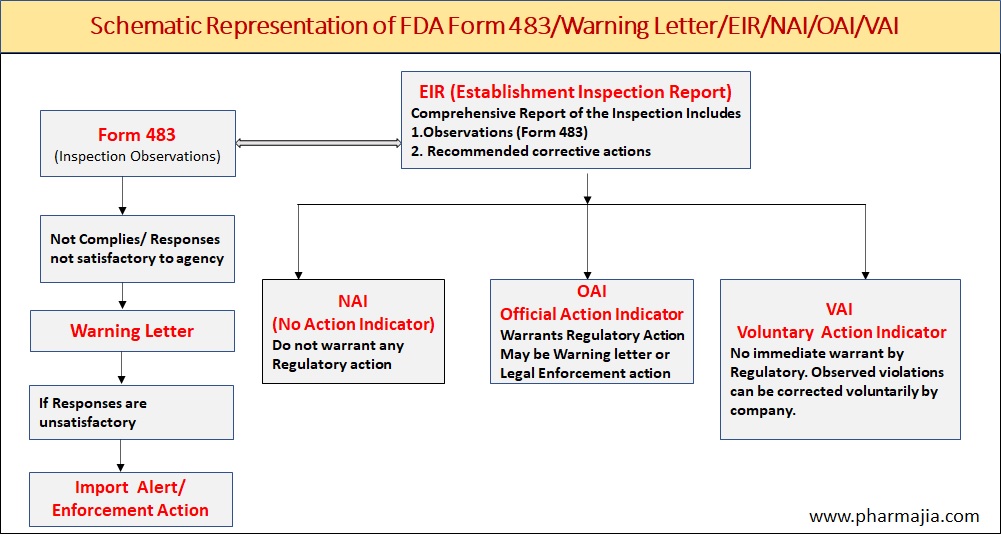
Form FDA 483 is a form used by the United States Food and Drug Administration (FDA) inspector to document and communicate any observations made during inspections of facilities that may be in violation of FDA regulations.
This form is issued to the company at the end of the inspection where potential violations that were observed during the inspection.
The purpose of the Form 483 is to notify the company of any potential issues and to request a written response from the company detailing how they plan to address the observations.
Response to the form 483 observations should be provided/submitted to FDA within 15 working days.
What is mean by Warning letters?
Warning letters are formal correspondence issued by regulatory agencies such as the United States Food and Drug Administration (FDA) to companies or individuals to communicate concerns and notify them of violations found during inspections or investigations.
After issuance of form FDA 483 to the Firm , FDA provide chance to that firm to provide responses to those issued FDA 483 observations and to explain the corrective and preventive actions taken by the firm
If FDA finds that the response to FDA 483 observations are not satisfactory and still if there is significant violations of the regulations and standards US FDA may issue warning letter to the firm based on nature of violations.
The purpose of a warning letter is to request that the recipient should take corrective action to address the identified violations and prevent similar violations from occurring in the future. Failure to adequately respond to a warning letter or to correct the identified violations may result in further enforcement action, such as fines, Import alert or legal action.
What is mean by EIR (Establishment Inspection Report) ?
EIR stands for Establishment Inspection Report. It is a document prepared by the United States Food and Drug Administration (FDA) after conducting an inspection of a regulated facility such as a drug manufacturing plant.
The EIR provides a comprehensive report of the inspection, including observations (Form FDA 483) made during the inspection, any deviations from the applicable regulations or standards, and any recommended corrective actions. The report also includes a classification of the inspection findings, such as “no action indicated,” “voluntary action indicated,” or “official action indicated,” which determines the level of follow-up action that may be taken by the FDA.
EIR (Establishment Inspection Report) classified further in below categories :
- No action Indicated (NAI)
- Official action Indicated (OAI)
- Voluntary action Indicated (VAI)
What is mean by No action Indicated (NAI) ?
No action indicated” is a classification used to indicate that no objectionable conditions or practices were found during an inspection of a regulated facility, or that any minor violations found do not warrant regulatory action.
This classification is used when the agency determines that the facility is in compliance with applicable laws, regulations, or standards, and that no further action is necessary at this time.
What is man by Official action Indicated (OAI) ?
Official action indicated” is a classification used to indicate that significant objectionable conditions or practices were found during an inspection of a regulated facility and warrants regulatory action.
Agency believes observed violations warrant regulatory action to ensure compliance with applicable laws, regulations, or standards and to protect the public. The regulatory action may include issuing a warning letter, initiating legal proceedings, or other enforcement actions.
The company is expected to respond in writing to the FDA with a plan to correct the identified violations and to follow through with the corrective actions. The FDA may conduct a follow-up inspection to ensure that the company has taken appropriate corrective actions. Failure to take adequate corrective action may result in further enforcement action by the FDA.
What is man by Voluntary action Indicated (VAI) ?
“Voluntary action indicated” is a classification used to indicate that, when the agency believes that the Observed violations do not warrant immediate regulatory action and violations can be corrected through voluntary action taken by the company.
The company is expected to respond in writing to the FDA with a plan to correct the identified violations and to follow through with the corrective actions. The FDA may conduct a follow-up inspection to ensure that the company has taken appropriate corrective actions.
Image I : Schematic Representation of Form FDA 483/Warning Letter/EIR/NAI/OAI/VAI.